
COLLABORATES WITH GOVERNMENT HEALTH INSTITUTIONS

Collaborates with Department of Medical Services, Ministry of Public Health to create an IMCRC responsible for research and development of medical cannabis formulations, products and delivery systems for domestic patients and future exports.

Collaborate with Medical Services Department of Bangkok Metropolitan Authority to develop medical cannabis products for MSD network of hospitals throughout Bangkok.
EASE LABS PHARMA

Ease Labs Pharma is the group’s pharmaceutical company
Using agile and innovative models, with a restless, questioning and investigative team, we bring a modern, fast-passed and cost-effective pharmaceutical model, launching innovative products connected to new health consumption trends.
Our GMP-Pharma facility located in Belo Horizonte – Brazil, has 3,000m2, two production lines, with the capacity to manufacture over 5M units of products and its own quality control labs (physical chemical and micro-biological) and R&D laboratory.



Avicanna and Ease Labs Pharma
Granted Commercialization Approval for Pharmaceutical Preparation in Brazil
The first THC containing pharmaceutical preparations produced in Brazil approved by the Brazilian Health Regulatory Agency (Agencia Nacional de Vigilancia Sanitaria, “ANVISA”)
TORONTO, Jan. 30, 2024 (GLOBE NEWSWIRE) — Avicanna Inc. (“Avicanna” or the “Company) (TSX: AVCN) (OTCQX: AVCNF) (FSE: ONN) a biopharmaceutical company focused on the development, manufacturing, and commercialization of plant- derived cannabinoid-based products is pleased to announce ANVISA has granted commercialization approval of Ease Labs Pharma (“Ease Labs”) pharmaceutical preparation produced in Brazil
EASE LABS BUSINESS VALUES
- Brazil's first Pharmaceutical Company specialized in Cannabis fully licensed by ANVISA
- 2nd Pharmaceutical Company to launch an Anvisa approved Cannabis Product formulated in Brazil
- Ease Labs products being sold in +1500 retail pharmacies across 23 states
- USD20m+ B2B Manufacture Agreement (take of pay)
- Unique positioning to supply to SUS (Brazilian Public Health System)
- Developed and registered Cannabis Products
- On-going clinical trials proving the application for targeted conditions
- License to dev nd distribute controlled and non-controlled drugs
- Unlimited exports
- Highly qualified sales force
- In-house quality control laboratory (physical-chemical and microbiological)
- Highly qualified technical team
- Active R&D with pipeline of potential patentable drugs
GMP CERTIFIED PHARMA PRODUCTIONS
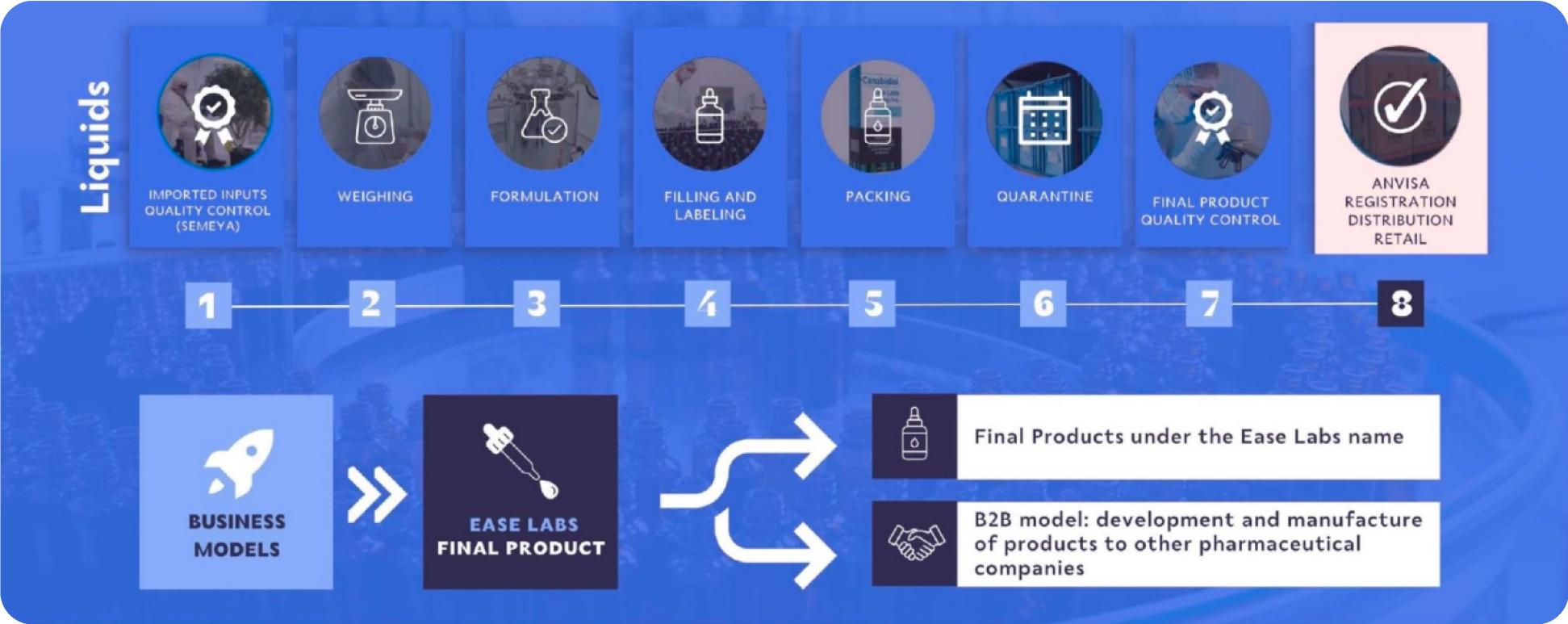
EASE LABS GROUP

Guslavo Palhares
CEO
Before founding Ease Labs in 2017, Gustavo acquired over 11 years of experience structuring international M&A deals as a lawyer in Washington
DC.
Gustavo holds a master’s degree in law from Northwestern University in Chicago-IL and took the postgraduate program at FGV-SP.
He is also licensed to practice law in Brazil and the United States, as an international lawyer recognized by the District of Columbia Bar Association.

Guilherme Franco
COO | CFO
Founder of Ease Labs in 2017, Guilherme holds a degree in Business Administration from IBMEC -MG. He has over 13 years financial market experience.
Former statutory director for operations, information technology security and product structuring with a financial institution in Brazil. Extensive business management experience, in particular non-financial holding companies as well as managing portfolios of assets, derivatives and investment funds.

Vitor Bertoncini
Exective Director of the Raia Drogasil group
Maketing
Marketing Director at RD Raia Drogasil (Brazil’s biggest largest pharmacy chain) since 2018, in charge of CX, CRM, Loyalty, Branding, Communication and Growth. Vitor has a 25-year career in marketing, trade marketing, strategy, digital and ecommerce with majors such as Whirlpool and Schindler. He holds a degree in Marketing and Advertising from ESPM, a postgraduate degree from FGV/EAESP and an MBA from Business School São Paulo, as well as a digital transformation course at HSM University and Hyper Island.

Gonzalo Grillo
Head of Healthcare for Alvarez & Marsal
Business Straetegies
Managing Director and Partner of Alvarez & Marsal Brasil, he has specialized in restructuring and transforming companies, crisis management and interim management, having acted on several occasions as CEO and CRO for major Brazilian and international companies. He started his career as an entrepreneur in his native Uruguay, where he co-founded Sea Krill SRL. His A&M experiences includes leading: Hospital São Lucas, Hospital Einstein, Oncoclinicas, Drogaria Onofre – CVS, Diaverum Argentina (healthcare), Obino & Coppel (retailing), Alumini & Fidens (engineering), Prumo (infrastructure), and Azul (airline). Gonzalo has advised professional shareholders such as: BTG Pactual, EIG Partners, TPG, Pátria, IFC, Guggenheim Partners, Harvard Management Company, Soros Quantum Fund, Icahn Enterprise, Actis and family groups

Dr.Jose Alexandre Crippa
Psychiatrist / Professor and Researcher at USP-RP
Medical Committee
Prof. Dr. José A Crippa is one of the leading researchers in the globe in the field of canabinoids. Currently he is a Full Professor and was the Head (2017-2019) of the Department of Neurosciences and Behavior, Faculty of Medicine, University of São Paulo (USP), Ribeirão Preto, São Paulo, Brazil. He is also a Cannabinoid Pharma Consultant with Global Experience in Pharmaceutical Drug Development having worked for major pharmaceutical and cannabis global companies. Dr Crippa was an Honorary Visiting Lecturer at the Institute of Psychiatry, King’s College London, UK (2009-2012). He is now the Co-Director of the Brazilian Cannabinoid Research Center. He helped the University São Paulo of Ribeirão Preto Medical School expand the clinical and basic scientist researchers in the cannabinoid field, conducting dozens of trials with canabinoid based drugs.

Luiz Pianowski
Formerly R&D director with Ache
P&D
Biochemical pharmacy degree and Pharmaceutical Technology PhD from Porto, Portugal. His portfolio includes more than 45 patents and 15 products on the market, such as: Acheflan, Sintocalmy, Prostokos, Giamebil etc. Acted as industrial director for Hebron pharmaceuticals in Caruaru for than 7 years. Subsequently, he was deputy R&D head at Laboratório Farmacêutico Aché in São Paulo. He has published several scientific papers. As R&D vice president for MCG Brasil, he will be launching products in Brazil and the US in the coming months, including an immunomodulator for several autoimmune diseases.
Clinical trials
Trials are being developed in partbnership with Profressor Crippa and his team.
He is a distinguished cannabinoid pharmaceutical consultant with global experience in pharmaceutical drug development and codirects the Brazilian Cannabinoid Research Center, known for pioneering medicinal cannabis research. His prolific work has resulted in over 150 cannabinoid-related articles and over 14,500 citations, making him a leading figure in the neurosciences. He has contributed to numerous CBD formulations, advised international debates on cannabis regulation, and received numerous awards for his impactful contributions.

Dr.Jose Alexandre Crippa
Psychiatrist / Professor and Researcher at USP-RP Medical Committee
HEALTH AUTHORITIES COLLABORATION

Department of Medical Services


Insitute Of Reseacrh & Technology Accessment



EarthSide Thailand is at the forefront of transforming agriculture and sustainable product development in Thailand through the utilization of hemp’s potential. Our initiative, is backed by rigorous scientific research and a dedicated team of seasoned hemp experts. By promoting the cultivation of industrial hemp, we seek to create a sustainable and profitable cycle that benefits local farmers, government revenues, the environment, and EarthSide
Key Areas
“Cleaner” Energy
– Greener Products
– Biodegradable & Decomposable
– Recyclability
– Carbon Sequestration (Carbon Credits)

EARTHSIDE (NEW INVESTMENT)

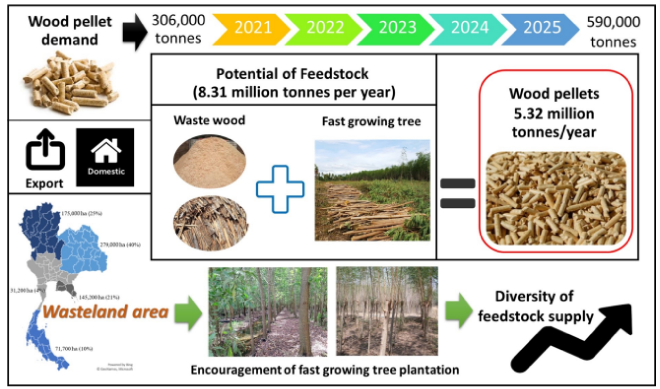
Thailand exported wood pellets less than our neightbour country (Malaysia & Vietnam) in 2015.
However, there is a huge demand of wood pellet (or alternative) over half a million tons by 2025,
another 8.3
Million tonnes of feedstock a huge potential for a fast growing trees in Thailand.
OTHER PRO DUCTS

Hemp Concrete Construction materials

Hemp Boards for Construction

Hemp Plastic Pellets

Hemp Utensils (Single use & multi-use)

Hemp eneygy pellets clean power

Hemp Packaging Food Packaging


